Because they are electronic, magnetic, and visual, organic-inorganic hybrid halides perovskite materials have gotten a lot of attention in solar study. People are interested in the magnetic qualities of perovskite materials, especially B-site replaced perovskite and perovskite manganite, because they are strong and have strong electron-electron interactions with the 3D manifold. It is possible for B-site modified perovskites to behave in a lot of different ways when different paramagnetic cations are combined at all three cation sites. Perovskite manganites have amazing multiferroic qualities, as well as magnetocaloric effect and magnetoresistance. They can be used in bioprocessing, magnetic cooling, and tiny magnetic sensor uses. The electronic structure of perovskite materials is mostly set by BX6 ions, while the unique electronic properties are caused by the lone pair of lead ions. Halide perovskites have grain borders that don’t carry electricity, which makes them a good candidate for making high-quality semiconductor films for a wide range of photoelectric devices.
Magnetic Properties
The discovery of B-site substituted perovskite oxides (A2B′B″X6) and nanoscale manganese-based perovskite oxides (AMnO3) has sparked a lot of interest in the magnetic properties of these materials. Perovskite materials are used in many things, like fuel cells, sensors, catalysts, magneto-optical materials, and more. Because they are strong and have strong electron-electron interactions with the 3D manifold, transition metal perovskite oxides have unique magnetic qualities.
c columnar order
The structure of perovskite is very flexible and has a good structure. Recently, one cation has been swapped out for half of the B-site cations. This has caused a double perovskite, A2BʲB″X6, to form. This structure makes perovskite products more flexible and complicated. The two different cations in the B-site create new and interesting combos, involving transition metals of the actinoides and lanthanoides types as well as main group elements.
The double perovskites are magnetically strong because they can hold different mixtures of paramagnetic cations in their cation sites in A2BʲB″X6. There are ferromagnetic (FM) and firimagnetic (FiM) compounds, as well as antiferromagnetic (AFM) compounds. Some of them have a magnetic sorting temperature (TC) that is very high. It’s very important how ordered the cations are, because spin-glass behaviour has been seen in several disorganised substances with strong long-range magnetic qualities.
The super-exchange interaction takes place over a pretty large area when there is only one paramagnetic B-site cation in the A2BʲB “X6 perovskite. When two atoms come together at 90°, they form a nearest neighbour (NN) interaction. When they come together at 180°, they form a next-nearest neighbour (NN) interaction. These two interactions can often be of the same order and can fight. What kind of paramagnetic ions are used determines how strong these reactions are.
When there are two magnetic B-site cations, they may interact with each other in a short-range B′–O–B″ way, as well as in a long-range B′–O–(B″)–O–B′ way and a B″–O–(B′)–O–B″ way. The orbital mixing between the transition metals and lantanoides is not strong enough for long-range superexchange interactions to control the magnetic order. The two B-site cation orbitals don’t always meet well in A2B′B″O6 perovskites. This means that the long-range B′–O–(B″)–O–B′ superexchange is often stronger than the short B′–O–B″ interaction in these compounds.
It is known that the A2B′BʺO6 perovskite has paramagnetic cations in the A-position. Compounds like Mn2+ or paramagnetic R in the A-position are examples of these. Most of the time, this interaction between cations in the A and B sites is weak. However, Pb in the A site can make the magnetic properties better because of super-exchange interactions of electrons in Pb 6s. In the A-site, the magnetic temps of A2BʲNbO6, A2NiReO6, A2MnWO6, and A2FeTiO6 are higher than those of Pb or Sr.
In A2B’BʺX6 perovskites, JT warping because of the d1 configuration has been seen once in a while. In Ba2SmMoO6, both JT distortion and TN show up at the same time. The relatively high value of TN = 130 K is caused by the way the orbitals are arranged. In Ba2NdMoO6, JT distortion changes the magnetic order, but there aren’t many connections between the magnetic order and the orbital.
The spin-orbit coupling and spin delocalisation chemically linked by the bond are much better in the 4d/5d metal than in the 3d metal. This means that the magnetic moment of the 4d/5d element is much lower. You can find Ir5+ compounds like Ba2HoIrO6 or R2NaIrO6 that don’t have magnetic ordering of the Ir sublattic or a lessened moment at Ir because they have a strong SOC.
The A2B′BʺX6 perovskites that have magnetic ordering have been reported to be AFM. More than 200 compounds have been reported to have AFM orders. Face-centered cubic (FCC) lattice is made up of a single B′ or B cation. When magnetic cations from this FCC array show up, they usually fall into three groups: I, II, and III.
On the one hand, magnetic qualities depend on how the orbitals of space ions overlap, and on the geometry of the overlap. In the case of A′AʺFeMoO6, the relationship between TC and bandwidth has been found where Aʺ = Ca, Ba, or Sr.
c type III
Because of the CMR, manganese oxides have a strong core electronic system, which gives them complex electronic and magnetic qualities. Manganese oxides have a lot of interesting properties, like phase separation, charge ordering (CO), and semi-metallicity. These properties make them one of the most interesting topics in condensed matter physics. They have also been used to make many prototype devices for science study and formulation technology.
Nanoscale perovskite manganite has a lot of room for growth and market potential in magnetism applications, such as bioprocessing, magnetic cooling, and tiny magnetic sensor applications. When perovskite manganite gets smaller until it’s only a few nanometres thick, its magnetic properties change a lot. For example, it loses a lot of its coercivity and gains surface spin-glass (SG). Scientists have seen the spin core-shell structure when the particle size drops to the nanometre level. The core has a lot of crystal flaws that make spinning less effective, and magnetic dead layers show up. The surface impact of the shell gets stronger as the particle size goes down, and the surface super-exchange interaction helps the FM core grow.
Nanosized FM manganite La0.67Ca0.33MnO3 (LCMO) has less saturation magnetisation and smaller grains. Scientists have come up with the core-shell model for nanoparticles. The core is naturally magnetic, and the magnetic qualities of the shell change things. The shell is about 3 nm thick, and along with the FM crystalline core, a magnetic dead layer has been found.
It has been found that the RCP values of 670, 533, 462, 535, and 511 J/kg are highest for mixtures of La0.845Sr0.155MnO3, La0.815Sr0.185MnO3, La0.7Ca0.25Sr0.05MnO3, Gd5Si2Ge2, and Pr0.63Sr0.37MnO3. The best RCP values have been seen in mixtures like HoMnO3, La0.6Ca0.4MnO3, Pr0.8Na0.2MnO3, Pr0.8Na0.15K0.05MnO3, Pr0.8Na0.1K0.1MnO3, and Pr0.8Na0.05K0.15MnO3.
Electronic Properties
The electronic structures of perovskites are set by BX6 blocks, especially close to the edge of the band. These special features are mostly caused by lone pairs of lead ions. The electron orbit of lead is 6 s below the top of the valence band (VB). It is safe for I and Pb to interact against each other in the valence band maximum (VBM), and the Pb state is what causes the conduction band minimum (CBM).
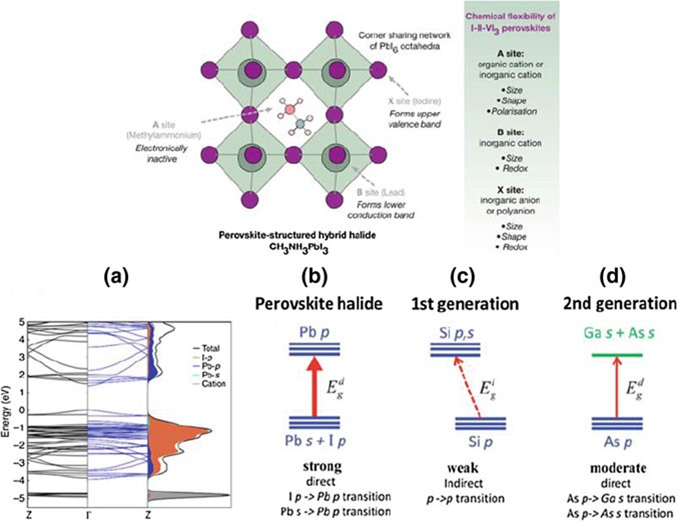
The perovskite material’s electric features can be changed by how the different ions are distributed in terms of density. The cation-related state density is not as high as VBM, so it doesn’t change the shape of the electronic bands. Perovskite materials have a reversed electronic band structure, which makes them better at absorbing light than first- and second-generation semiconductors. They are straight bandgap semiconductors that have a better chance of transitioning than others.
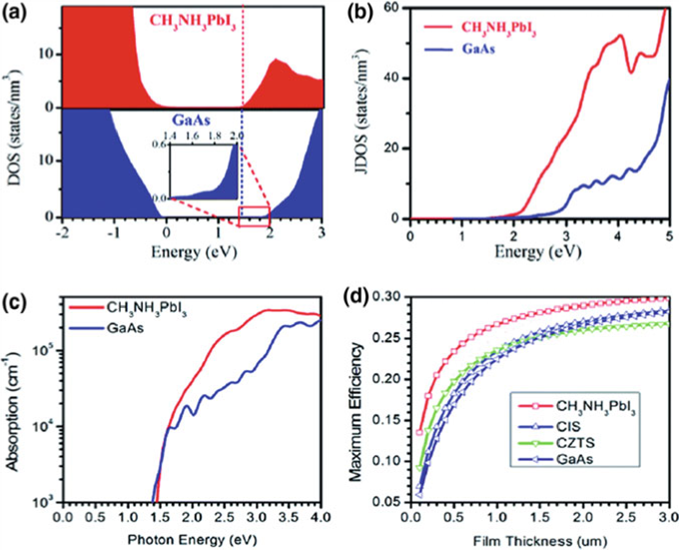
CBMs are marked in dashed lines, b Joint density of states of MAPbI3 and GaAs, c optical
absorptions of MAPbI3 and GaAs, d maximum efficiencies calculated for MAPbI3, CIS, CZTS,
and GaAs as a function of film thickness
The straight bandgap of both MAPbI3 and GaAs is the same, but their electronic structures are very different. The Pb b band gives perovskite material its bottom end of the conduction band (CB), while the delocalised s orbit of GaAs gives it its upper end. The change in bands between CB and VB comes from the mixed halides of Pb s and I p to Pb p orbitals. The possible change from Pb s to Pb p is higher than in GaAs because perovskites are better at absorbing light.
Perovskite polycrystalline films have grain borders (GBs) that are electrically neutral. These GBs are typically not visible but improve the performance of devices. When Pb s and I p interact in a stable way, the VBM goes up. This means that there are fewer flaw states and charge carriers recombining. Perovskite has good carrier transfer and carrier collection qualities, which is good for how well electronics work.
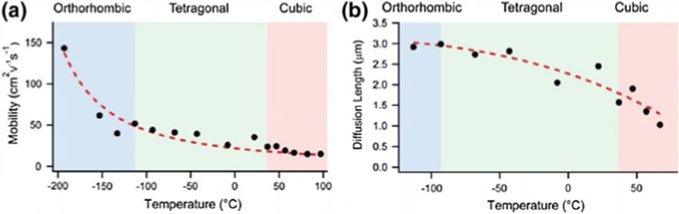
Solution method engineering can be used to make high-quality perovskite films. For example, optimised depositing techniques can be used to make the MAPbI3 thin film more mobile. The way the perovskite material is made changes the length of its spread.
| Compound (Perovskite Thin Films) | Measurement technique | Mobility (l) (cm2 v−1 s−1) | Diffusion length, LD (lm) | Charge lifetime, s (ns) | |
| MAPbI3 | PLQ | 0.66 | 0.13 | 9.6 | |
| PLQ | 1.4 | 0.13 | 4.5 | ||
| TRTS | 8.2 | 1.2 | 67 | ||
| MAPbI3−xClx | PLQ | 1.6 | 1.07 | 273 | |
| TRTS | 11.6 | 2.4 | 200 | ||
| MAPbBr3 | PLQ | 8.9 | 1.06 | 51 | |
| CH(NH2)2PbI3 | PLQ | 0.16 | 0.18 | 75 | |
| TRTS | 27 | 3.1 | 140 | ||
| CH(NH2)2PbBr3 | TRTS | 14 | 1.3 | 50 | |
| MASnI3 | TRTS | 1.6 | 0.03 | 0.2 |
Optical Properties
Scientists are studying perovskites to find ways to use them in solar cells, light monitors, and light-emitting diodes (LEDs). Because they have great optical qualities, like high absorption coefficients and direct bandgaps that can be changed, they are perfect for materials that absorb light and materials that release light. Because organic-inorganic hybrid perovskites have high absorption coefficients, it is possible to make the TiO2 layer thinner in perovskite. This means that the TiO2 layer thickness is lower than in solid-state dye sensitised solar cells.
Based on estimates by Shockley and Queisser (SQ), the highest amount of solar energy that can be converted into electricity using single-junction solar cells is about 33% at a 1.4 eV absorber energy bandgap. Single-junction solar cells, on the other hand, don’t work as well as they could because of problems like flaws, impurities, and interface potential barriers. If the bandgap of absorbing materials moves away from its ideal value of 1.4 eV, the maximum power conversion efficiency will go down.
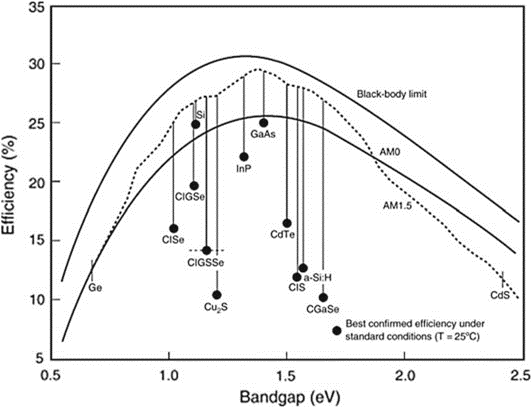
efficiencies for several solar cell technologies
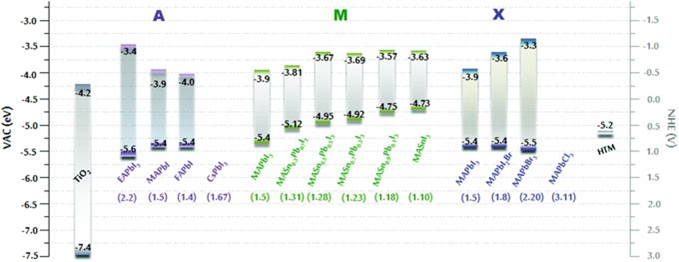
You can write perovskite materials as ABX3, where X is an ion and A and B are cations of different sizes. The bigger cation in organic-inorganic halide perovskites is always an organic group, and B is always either Pb2+ or Sn2+. The X anion is a halogen, and the most common ones are iodine (I−), bromine (Br−), and chlorine (Cl−).
The fact that perovskite can change the optical energy band through all of these A, B, and X combinations shows how versatile it is. In terms of how symmetrical they are, bulk perovskite shapes come in three types: cubic, tetragonal, and orthorhombic. The cation A is a key part of the phase structure of perovskites based on the empirical Goldschmidt tolerance factor theory. The bandgap usually changes at the same time.
It is first thought that tin (Sn), which is in the same family as lead (Pb), could be used instead. The bandgap of MASn1−xPbxI3 can be set between 1.17 and 1.55 eV, and the absorption spectrum goes from visible to near-infrared. This was found by Ogomi et al. In Hao et al.’s first test of the lead-free MASnI3 perovskite solar cell, the bandgap was lowered by 1.3 eV, which led to a PCE of over 5%. But the hardest part of working with MASn1−xPbxI3 or MASnI3 perovskite is still not fixed. This is because it is hard to keep Sn from oxidising to SnI4 in the iodide perovskite.
2′, 7, 7′-tetrakis-(N, N-dimethoxyphenyl-amine-)-9, 9′-spirobifluorene (spiro-MeOTAD). The
values under the chemical formula are the corresponding bandgap in eV
Perovskite materials, like quantum dots (QDs) and two-dimensional (2D) nanowires, are different from three-dimensional (3D) materials in how they reflect light. These materials have changeable absorption edges and photoluminescence peak places. They are also more stable in air and light and can handle defects. Even though perovskite quantum dots have natural flaws in their structure, they have special visual qualities.
Compared to the CIE, perovskite colloidal nanoparticles (NCs) have colours that are much more intense. The photoluminescence peaks of these NCs move continuously as the make-up of X and A, as well as their size and shape, is changed. Like in CH3NH3PbX3 NCs, the wide colour gamut and flexible colour range are good things.
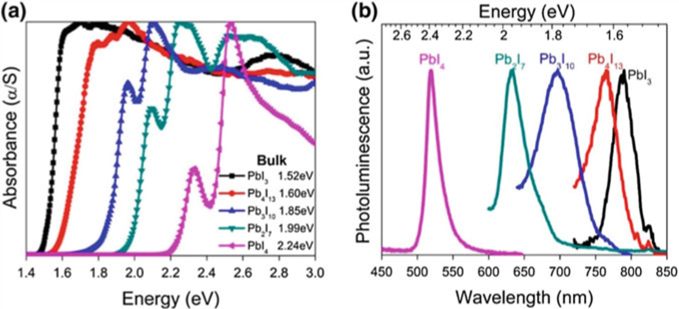
2D-perovskites are different from 3D-perovskites because they are made up of layers that are separated by biological gaps. 2D-perovskites are made up of AnAʈn−1MnX3n+1, where A and A’ are cations, M is a metal, and X is a halide. The quantum well width and optical bandgap are both based on the number n.
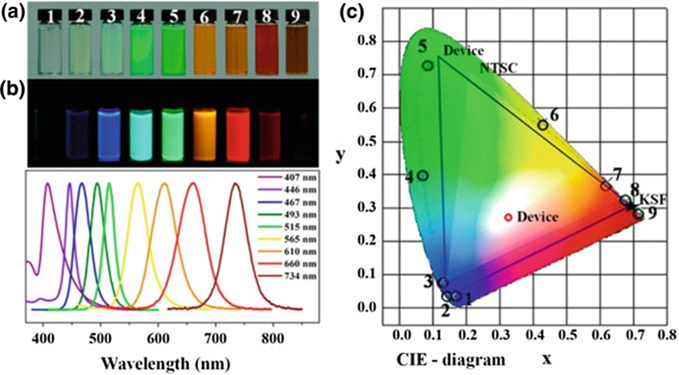
spectra (b) of composition-tuned CH3NH3PbX3 NCs. c PL spectra of CH3NH3PbX3 NCs plotted
on CIE chromaticity coordinates (no. 1–9, black circle) compared with common color standards
(pc-WLED devices (blue lines), and NTSC standard (bright area))
Researchers have discovered that a well-designed 2D/3D hybrid perovskite can combine the best features of both 2D- and 3D-perovskite, offering good photo-electronic qualities and resistance to moisture. If you can change this, it could help with multijunction perovskite solar cells or hybrid tandem solar cells with thin films of silicon or copper indium gallium diselenide.
MAPbI3 and (BA)2(MA)n–1PbnI3n+1 with the value of n varying from 1 to 4

Leave a Reply